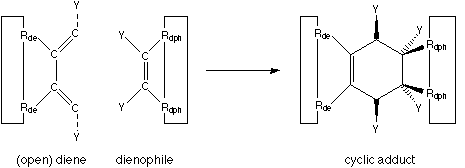
FIGURE 2: Diels-Alder cycloaddition.
The big benefits of a Diels-Alder reaction are: no leaving group is generated, nothing ionic is involved (which means that the reaction rate is little influenced by the dielectric constant of the surrounding environment), and it works in solvents from hexane to water. The two components of the Diels-Alder reaction can be viewed as two parts which simply snap together when held in sufficient proximity. The reactivity can be tailored by the appropriate attachment of electron-withdrawing and electron-supplying neighbouring functional groups (denoted as "Y" in figure 2).